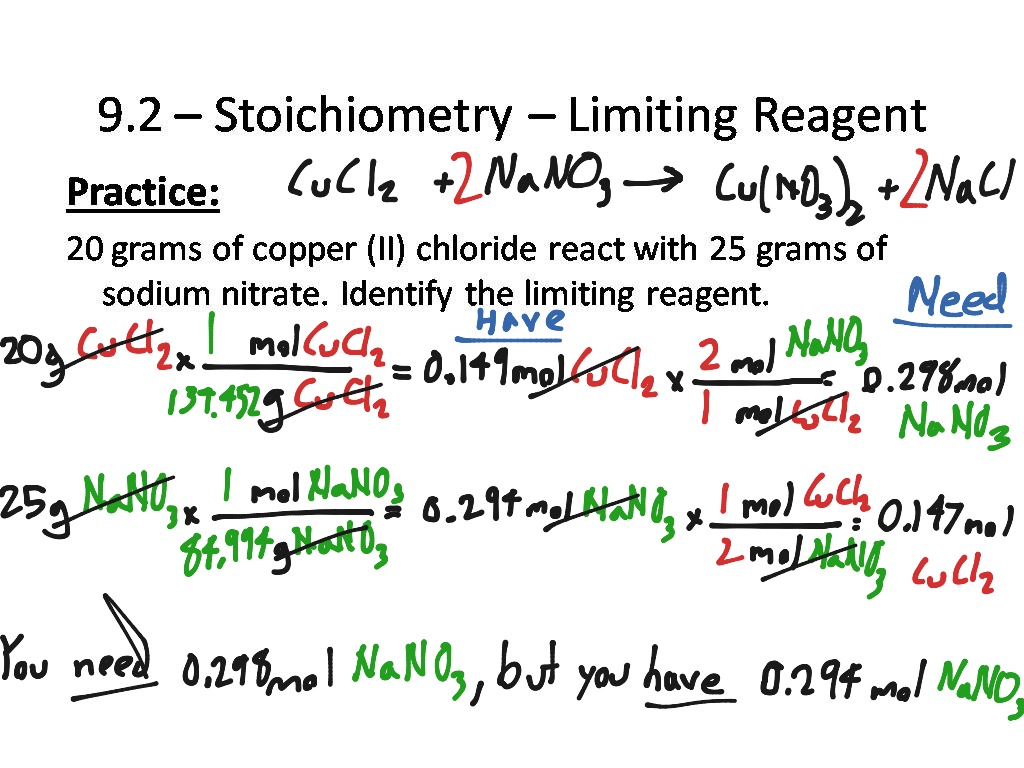What Is Limiting Reagent In Organic Chemistry . It limits the reaction from continuing because there is. Identify a limiting reagent from a set of reactants. Thus, the limiting reagent determines when to. a reactant in a chemical reaction that determines the amount of product that is produced is the limiting reactant or limiting reagent. the reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. a limiting reagent or limiting reactant is a substance that has been wholly consumed in a chemical reaction. the limiting reagent is the one that is totally consumed; The other reactant or reactants are considered to be in excess. The reason for a limiting reactant is that elements and compounds react in a balanced chemical equation according to the mole ratio between them. define and determine theoretical yields, actual yields, and percent yields. the reactant you run out of is called the limiting reagent;
from www.showme.com
It limits the reaction from continuing because there is. a limiting reagent or limiting reactant is a substance that has been wholly consumed in a chemical reaction. the limiting reagent is the one that is totally consumed; The other reactant or reactants are considered to be in excess. Thus, the limiting reagent determines when to. the reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. The reason for a limiting reactant is that elements and compounds react in a balanced chemical equation according to the mole ratio between them. the reactant you run out of is called the limiting reagent; a reactant in a chemical reaction that determines the amount of product that is produced is the limiting reactant or limiting reagent. Identify a limiting reagent from a set of reactants.
9.2 Stoichiometry Limiting Reagent Science, Chemistry ShowMe
What Is Limiting Reagent In Organic Chemistry a reactant in a chemical reaction that determines the amount of product that is produced is the limiting reactant or limiting reagent. a reactant in a chemical reaction that determines the amount of product that is produced is the limiting reactant or limiting reagent. a limiting reagent or limiting reactant is a substance that has been wholly consumed in a chemical reaction. the reactant you run out of is called the limiting reagent; the limiting reagent is the one that is totally consumed; The other reactant or reactants are considered to be in excess. the reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. It limits the reaction from continuing because there is. Thus, the limiting reagent determines when to. Identify a limiting reagent from a set of reactants. define and determine theoretical yields, actual yields, and percent yields. The reason for a limiting reactant is that elements and compounds react in a balanced chemical equation according to the mole ratio between them.
From www.youtube.com
Limiting Reagent Chemistry Tutorial YouTube What Is Limiting Reagent In Organic Chemistry The reason for a limiting reactant is that elements and compounds react in a balanced chemical equation according to the mole ratio between them. a reactant in a chemical reaction that determines the amount of product that is produced is the limiting reactant or limiting reagent. define and determine theoretical yields, actual yields, and percent yields. It limits. What Is Limiting Reagent In Organic Chemistry.
From chemistry.analia-sanchez.net
Limiting Reactant Notes Chemistry Classes / Ronald Reagan S.H.S. What Is Limiting Reagent In Organic Chemistry a limiting reagent or limiting reactant is a substance that has been wholly consumed in a chemical reaction. the limiting reagent is the one that is totally consumed; Identify a limiting reagent from a set of reactants. the reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant.. What Is Limiting Reagent In Organic Chemistry.
From classmediaugscondonable.z13.web.core.windows.net
How To Determine Limiting And Excess Reactant What Is Limiting Reagent In Organic Chemistry a reactant in a chemical reaction that determines the amount of product that is produced is the limiting reactant or limiting reagent. a limiting reagent or limiting reactant is a substance that has been wholly consumed in a chemical reaction. Thus, the limiting reagent determines when to. the reactant you run out of is called the limiting. What Is Limiting Reagent In Organic Chemistry.
From chemistryguru.com.sg
Determine Limiting Reagent What Is Limiting Reagent In Organic Chemistry Thus, the limiting reagent determines when to. The reason for a limiting reactant is that elements and compounds react in a balanced chemical equation according to the mole ratio between them. the reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. It limits the reaction from continuing because there. What Is Limiting Reagent In Organic Chemistry.
From exofcvqrg.blob.core.windows.net
Crushing A Solid Reactant Into A Powder Will at William Hays blog What Is Limiting Reagent In Organic Chemistry the limiting reagent is the one that is totally consumed; Thus, the limiting reagent determines when to. the reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Identify a limiting reagent from a set of reactants. It limits the reaction from continuing because there is. The reason for. What Is Limiting Reagent In Organic Chemistry.
From medium.com
Experiment 5 Synthetic Chemistry Erwin Rem. Medium What Is Limiting Reagent In Organic Chemistry It limits the reaction from continuing because there is. Identify a limiting reagent from a set of reactants. The other reactant or reactants are considered to be in excess. a limiting reagent or limiting reactant is a substance that has been wholly consumed in a chemical reaction. the reactant you run out of is called the limiting reagent;. What Is Limiting Reagent In Organic Chemistry.
From printablevogler.z4.web.core.windows.net
Limiting Reactant Worksheet With Answers Pdf What Is Limiting Reagent In Organic Chemistry The reason for a limiting reactant is that elements and compounds react in a balanced chemical equation according to the mole ratio between them. the reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. Identify a limiting reagent from a set of reactants. define and determine theoretical yields,. What Is Limiting Reagent In Organic Chemistry.
From www.researchgate.net
List of chemicals and reagents used in the study. Download Table What Is Limiting Reagent In Organic Chemistry Identify a limiting reagent from a set of reactants. the reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. a limiting reagent or limiting reactant is a substance that has been wholly consumed in a chemical reaction. The reason for a limiting reactant is that elements and compounds. What Is Limiting Reagent In Organic Chemistry.
From learningzoneemplegadafx.z14.web.core.windows.net
How To Determine Limiting And Excess Reactant What Is Limiting Reagent In Organic Chemistry the reactant you run out of is called the limiting reagent; a reactant in a chemical reaction that determines the amount of product that is produced is the limiting reactant or limiting reagent. Thus, the limiting reagent determines when to. the limiting reagent is the one that is totally consumed; a limiting reagent or limiting reactant. What Is Limiting Reagent In Organic Chemistry.
From chemistry.analia-sanchez.net
Limiting Reactant Notes Chemistry Classes / Ronald Reagan S.H.S. What Is Limiting Reagent In Organic Chemistry a limiting reagent or limiting reactant is a substance that has been wholly consumed in a chemical reaction. It limits the reaction from continuing because there is. Identify a limiting reagent from a set of reactants. a reactant in a chemical reaction that determines the amount of product that is produced is the limiting reactant or limiting reagent.. What Is Limiting Reagent In Organic Chemistry.
From studydbderrico.z13.web.core.windows.net
Chemsheets Limiting Reagents 1 What Is Limiting Reagent In Organic Chemistry Identify a limiting reagent from a set of reactants. a reactant in a chemical reaction that determines the amount of product that is produced is the limiting reactant or limiting reagent. the reactant you run out of is called the limiting reagent; It limits the reaction from continuing because there is. a limiting reagent or limiting reactant. What Is Limiting Reagent In Organic Chemistry.
From webapi.bu.edu
💄 What is the limiting reactant in a chemical reaction. Limiting What Is Limiting Reagent In Organic Chemistry The reason for a limiting reactant is that elements and compounds react in a balanced chemical equation according to the mole ratio between them. Identify a limiting reagent from a set of reactants. The other reactant or reactants are considered to be in excess. the reactant you run out of is called the limiting reagent; Thus, the limiting reagent. What Is Limiting Reagent In Organic Chemistry.
From learningisidro.z13.web.core.windows.net
How To Work Out The Limiting Reactant What Is Limiting Reagent In Organic Chemistry The reason for a limiting reactant is that elements and compounds react in a balanced chemical equation according to the mole ratio between them. Identify a limiting reagent from a set of reactants. The other reactant or reactants are considered to be in excess. a reactant in a chemical reaction that determines the amount of product that is produced. What Is Limiting Reagent In Organic Chemistry.
From worksheetmediabocca.z13.web.core.windows.net
Identify The Limiting Reagent If Any What Is Limiting Reagent In Organic Chemistry a limiting reagent or limiting reactant is a substance that has been wholly consumed in a chemical reaction. The reason for a limiting reactant is that elements and compounds react in a balanced chemical equation according to the mole ratio between them. the reactant you run out of is called the limiting reagent; a reactant in a. What Is Limiting Reagent In Organic Chemistry.
From www.chemistrylearner.com
Limiting Reagent (Reactant) Definition, Examples, and Problems What Is Limiting Reagent In Organic Chemistry the reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. a reactant in a chemical reaction that determines the amount of product that is produced is the limiting reactant or limiting reagent. Identify a limiting reagent from a set of reactants. a limiting reagent or limiting reactant. What Is Limiting Reagent In Organic Chemistry.
From www.pinterest.com.mx
Limiting Reactant Worksheet Answers Beautiful Limiting Reagent What Is Limiting Reagent In Organic Chemistry The reason for a limiting reactant is that elements and compounds react in a balanced chemical equation according to the mole ratio between them. a limiting reagent or limiting reactant is a substance that has been wholly consumed in a chemical reaction. The other reactant or reactants are considered to be in excess. Thus, the limiting reagent determines when. What Is Limiting Reagent In Organic Chemistry.
From www.showme.com
9.2 Stoichiometry Limiting Reagent Science, Chemistry ShowMe What Is Limiting Reagent In Organic Chemistry the reactant you run out of is called the limiting reagent; the reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. define and determine theoretical yields, actual yields, and percent yields. a reactant in a chemical reaction that determines the amount of product that is produced. What Is Limiting Reagent In Organic Chemistry.
From eduinput.com
What is the limiting reactant? Explained with Examples What Is Limiting Reagent In Organic Chemistry define and determine theoretical yields, actual yields, and percent yields. Thus, the limiting reagent determines when to. a limiting reagent or limiting reactant is a substance that has been wholly consumed in a chemical reaction. the reactant that is consumed first and limits the amount of product(s) that can be obtained is the limiting reactant. the. What Is Limiting Reagent In Organic Chemistry.